An oxidation reaction involves the loss of electrons by a substance, leading to an increase in its oxidation state. Commonly observed in reactions with oxygen or other electronegative elements, oxidation reactions often result in the formation of oxides.
They play a crucial role in various chemical processes, including combustion, corrosion, and redox reactions, influencing the transformation of substances in different environments.
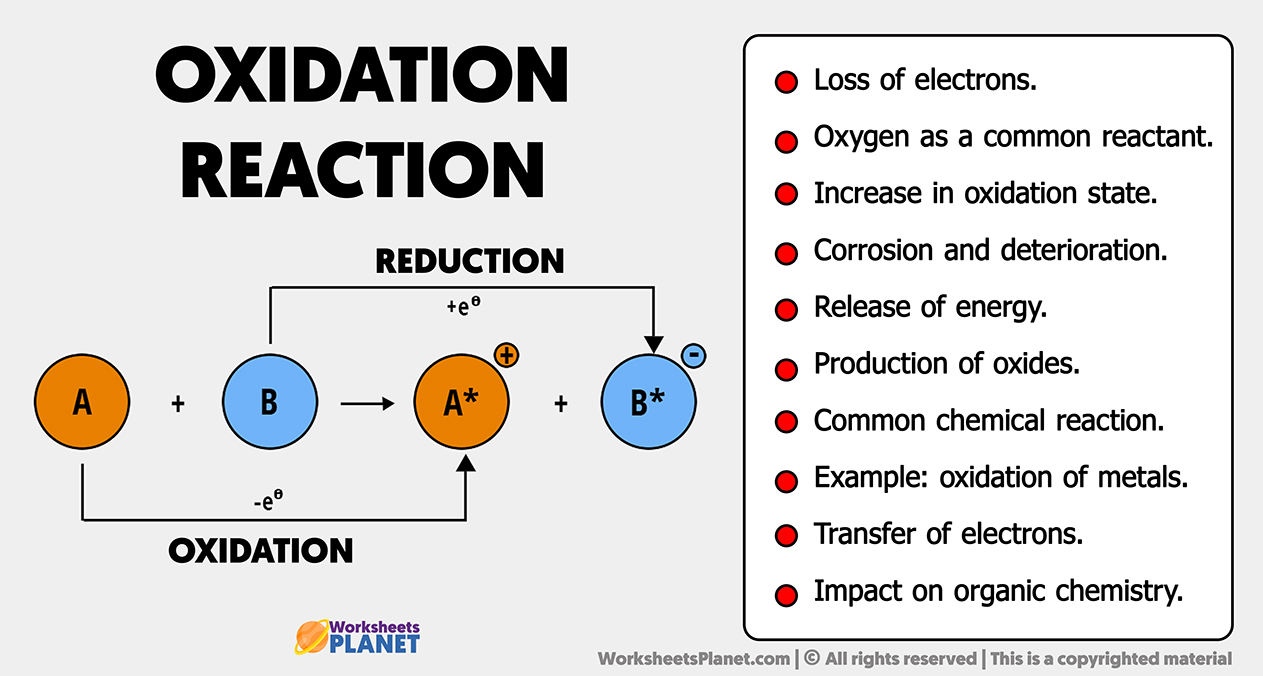
- Loss of electrons.
- Oxygen as a common reactant.
- Increase in oxidation state.
- Corrosion and deterioration.
- Release of energy.
- Production of oxides.
- Common chemical reaction.
- Example: oxidation of metals.
- Transfer of electrons.
- Impact on organic chemistry.

